Abbott gets FDA approval for its dissolvable stent Absorb
by Gail Kalinoski, Contributing Reporter | July 06, 2016
Business Affairs
Cardiology
Risk Management
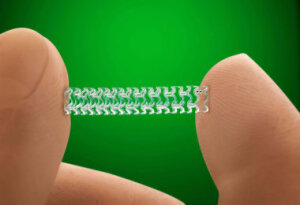
Price will be 'modestly higher'
than conventional stents
than conventional stents
The first fully absorbable stent to treat coronary disease – Absorb by Abbott Laboratories – has been approved by the U.S. Food and Drug Administration as an alternative to metal heart stents.
Manufactured by Abbott Vascular of Santa Clara, Calif., the full name of the stent is the Absorb GT1 Bioresorbable Vascular Scaffold System (BVS). It is made from a biodegradable polymer that is similar to other types of absorbable medical devices, such as sutures.
The device, which releases the drug everolimus to limit the growth of scar tissue, is gradually absorbed by the body in about three years. It eliminates the presence of foreign material in the artery once the stent is no longer needed.
The approval comes nearly four months after the device received a positive review from an FDA advisory panel that ruled the benefits outweighed the risks. The panel of independent experts had reviewed clinical data about the safety and efficacy of Absorb comparing it to the leading metallic drug-eluting stent that is a permanent device.
“The FDA’s approval of the Absorb GT1 BVS offers a new treatment option for individuals who are candidates for angioplasty, but would prefer an absorbable device rather than a permanent metallic coronary stent,” Dr. Bram Zuckerman, director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health, said in a prepared statement.
The FDA said it had evaluated data from a randomized trial of 2,008 patients, which compared the rate of major adverse cardiac events between those receiving the Absorb device and those getting the metallic stents. After one year, the Absorb GT1 BVS group showed a major cardiac event rate of 7.8 percent, which was clinically compared to the rate of 6.1 percent in the control group. Also, after one year, the rate of blood clots forming within the devices was 1.54 percent for the Absorb GT1 BVS group and 0.74 percent for those in the control group, according to the FDA.
“The Absorb bioresorbable scaffold represents a major advance in the treatment of coronary artery disease," Dr. Gregg W. Stone, FACC, FSCAI, director, cardiovascular research and education, Center for Interventional Vascular Therapy, Columbia University Medical Center, New York-Presbyterian Hospital and the chairman of the ABSORB clinical trial program, said in an Abbott news release.
“This novel technology appeals to both physicians and patients alike because after treating the underlying blockage it is completely absorbed, leaving nothing behind. No metal means the treated artery can pulse and flex naturally as demands on the heart change with everyday activities. No metal may also reduce the potential of future blockages that occur with permanent metallic stents, and allows easier access to other treatment options should they prove necessary in the patient's future,” he said.
Abbott plans to offer the Absorb device, which had already been available in Europe, Latin America and several Asian markets, to hospitals in the United States, starting with the centers that participated in the Absorb clinical trials. It has been used to treat more than 150,000 people around the world with heart disease.
Abbott is introducing the Absorb device to the U.S. market in phases, according to the Wall Street Journal, in part because of some concerns about patients with small vessels being more prone to suffering clots.
The company did not release a price for Absorb, but told the WSJ it expects the absorbable stent will “only be modestly higher” than its metal stents.
Coronary artery disease reportedly affects 15 million people the U.S. alone.
Manufactured by Abbott Vascular of Santa Clara, Calif., the full name of the stent is the Absorb GT1 Bioresorbable Vascular Scaffold System (BVS). It is made from a biodegradable polymer that is similar to other types of absorbable medical devices, such as sutures.
The device, which releases the drug everolimus to limit the growth of scar tissue, is gradually absorbed by the body in about three years. It eliminates the presence of foreign material in the artery once the stent is no longer needed.
The approval comes nearly four months after the device received a positive review from an FDA advisory panel that ruled the benefits outweighed the risks. The panel of independent experts had reviewed clinical data about the safety and efficacy of Absorb comparing it to the leading metallic drug-eluting stent that is a permanent device.
“The FDA’s approval of the Absorb GT1 BVS offers a new treatment option for individuals who are candidates for angioplasty, but would prefer an absorbable device rather than a permanent metallic coronary stent,” Dr. Bram Zuckerman, director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health, said in a prepared statement.
The FDA said it had evaluated data from a randomized trial of 2,008 patients, which compared the rate of major adverse cardiac events between those receiving the Absorb device and those getting the metallic stents. After one year, the Absorb GT1 BVS group showed a major cardiac event rate of 7.8 percent, which was clinically compared to the rate of 6.1 percent in the control group. Also, after one year, the rate of blood clots forming within the devices was 1.54 percent for the Absorb GT1 BVS group and 0.74 percent for those in the control group, according to the FDA.
“The Absorb bioresorbable scaffold represents a major advance in the treatment of coronary artery disease," Dr. Gregg W. Stone, FACC, FSCAI, director, cardiovascular research and education, Center for Interventional Vascular Therapy, Columbia University Medical Center, New York-Presbyterian Hospital and the chairman of the ABSORB clinical trial program, said in an Abbott news release.
“This novel technology appeals to both physicians and patients alike because after treating the underlying blockage it is completely absorbed, leaving nothing behind. No metal means the treated artery can pulse and flex naturally as demands on the heart change with everyday activities. No metal may also reduce the potential of future blockages that occur with permanent metallic stents, and allows easier access to other treatment options should they prove necessary in the patient's future,” he said.
Abbott plans to offer the Absorb device, which had already been available in Europe, Latin America and several Asian markets, to hospitals in the United States, starting with the centers that participated in the Absorb clinical trials. It has been used to treat more than 150,000 people around the world with heart disease.
Abbott is introducing the Absorb device to the U.S. market in phases, according to the Wall Street Journal, in part because of some concerns about patients with small vessels being more prone to suffering clots.
The company did not release a price for Absorb, but told the WSJ it expects the absorbable stent will “only be modestly higher” than its metal stents.
Coronary artery disease reportedly affects 15 million people the U.S. alone.
You Must Be Logged In To Post A CommentRegisterRegistration is Free and Easy. Enjoy the benefits of The World's Leading New & Used Medical Equipment Marketplace. Register Now! |
|









